According to Goldstein , Atoms are electrically neutral in nature, should necessarily possess positively charged particles to balance the negatively charged electrons.So, The presence of positive charged particles in the atom has been predicted by Goldstein.
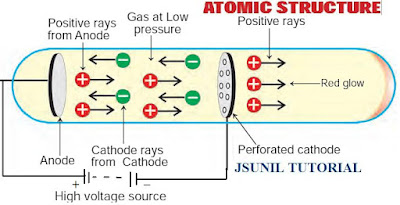
Goldstein’s Experiment: Goldstein repeated the cathode ray experiment by using a perforated
cathode. On applying a high voltage under low pressure, he observed a faint red glow on the wall behind the cathode.
Since these rays originated from the anode, they were called anode rays or canal rays or positive rays.
Anode rays were found as a stream of positively charged particles.
When hydrogen gas is taken in a discharge tube, the positively charged particles obtained from hydrogen gas are called PROTONS.
Each of these protons is produced when one electron is removed from one hydrogen atom.
Thus, proton can be defined as hydrogen ion (H+)
Properties of anode rays
1. Anode rays travel in straight lines
2. Anode rays consist of material particles since they rotate the light paddle wheel placed in their path.
3. Anode rays are deflected by electric and magnetic field since they deflect towards negatively charged plate. This shows that anode rays consist of positively charged particles.
4. The properties of anode rays depend upon the nature of gas taken in the discharge tube.
Atom model is the description of depicting the arrangement of various fundamental particles inside the atom.
Thomson’s atomic model:
According to J.J. Thomson Limitation of Thomson model :Thomson’s model could successfully explain the electrical neutrality of atom. However, it failed to explain how the positively charged particles are shielded from the negatively charged electrons without getting neutralized.
Limitation of Thomson model :Thomson’s model could successfully explain the electrical neutrality of atom. However, it failed to explain how the positively charged particles are shielded from the negatively charged electrons without getting neutralized.
Isobars : The atoms of different elements with same mass number but different atomic numbers are called “Isobars”.
Atomic weight: The atomic weight of an element is the average weight of all the isotopes of that element. It is usually expressed as gram atomic weight.
Goldstein’s Experiment: Goldstein repeated the cathode ray experiment by using a perforated
cathode. On applying a high voltage under low pressure, he observed a faint red glow on the wall behind the cathode.
Since these rays originated from the anode, they were called anode rays or canal rays or positive rays.
Anode rays were found as a stream of positively charged particles.
When hydrogen gas is taken in a discharge tube, the positively charged particles obtained from hydrogen gas are called PROTONS.
Each of these protons is produced when one electron is removed from one hydrogen atom.
H ---------> H+ + e
Anode rays cathode rays
Thus, proton can be defined as hydrogen ion (H+)
Properties of anode rays
1. Anode rays travel in straight lines
2. Anode rays consist of material particles since they rotate the light paddle wheel placed in their path.
3. Anode rays are deflected by electric and magnetic field since they deflect towards negatively charged plate. This shows that anode rays consist of positively charged particles.
4. The properties of anode rays depend upon the nature of gas taken in the discharge tube.
5. The mass of the particle is the same as the atomic mass of the gas inside the discharge tube.
The study of the properties of the fundamental particles, like electron and proton, led to the conception of various atom models.Atom model is the description of depicting the arrangement of various fundamental particles inside the atom.
Thomson’s atomic model:
According to J.J. Thomson
 Limitation of Thomson model :Thomson’s model could successfully explain the electrical neutrality of atom. However, it failed to explain how the positively charged particles are shielded from the negatively charged electrons without getting neutralized.
Limitation of Thomson model :Thomson’s model could successfully explain the electrical neutrality of atom. However, it failed to explain how the positively charged particles are shielded from the negatively charged electrons without getting neutralized.- The nucleus is made up of protons and neutrons. These are bound together by a strong nuclear force.
- Electrons and protons carry equal but opposite charges. In a neutral atom, the number of electrons is the same as the number of protons.
- Electrons orbit the nucleus at certain fixed levels called shells.
2. The positive and negative charges are equal in magnitude hence the atom as a whole is electrically neutral.
Thomson’s model of atom is popularly known as plum pudding or apple pie model
Neutron: The neutral fundamental particle present in the atom is called neutron.
The mass of a neutron is 1.00898 amu or 1.67495 X 10-27kg. It has no charge.
Discovery of neutrons: Chadwick discovered neutrons during the bombardment of a thin layer of 4Be9 with α-particles.
In 1932, James Chadwick bombarded beryllium (Be) with alpha aprticles. He allowed the radiation emitted by beryllium to incident on a paraffin wax. It was found that protons were shot out form the paraffin wax. People began to look for what was in the "beryllium radiations"
This discovery of neutron marked the beginning of current theories of nuclear structure. Immediately, the neutron-proton model (the Rutherford-Bohr model) of the nucleus was adopted:
AOMIC NUMBER, MASS NUMBER, ISOTOPES, ISOBARS, ATOMIC WEIGHT
Atomic number (Z):The number of protons or the number of electrons in an atom is called atomic number. It is represented by 'Z'.
Mass number(A): The total number of protons and neutrons in an atom is called mass number. It is denoted by 'A'.
Mass number(A): The total number of protons and neutrons in an atom is called mass number. It is denoted by 'A'.
A = no. of protons + no. of neutrons
A = Z + no. of neutrons no. of neutrons = A - Z
Isotope: Isotopes are the atoms of an element with same atomic number but differ in their mass numbers i.e., the isotopes of an element have same number of protons but differ in the number of neutrons.
E.g. Hydrogen has three isotopes :
Hydrogen(1H1),
Deuterium (1H2) and
Tritium (1H3).
They have same number of protons (= 1) but the numbers of neutrons are 1, 2 and 3 respectively.
E.g. 6C14, 7N14
Atomic weight: The atomic weight of an element is the average weight of all the isotopes of that element. It is usually expressed as gram atomic weight.
Note: The atomic number is a whole number whereas the atomic weight may be a fractional number.
Related Post -Structure of Atom: IX
Related Post -Structure of Atom: IX
|
Visit to Download Related Post -Structure of Atom
|
|
|
Structure of Atom: IX NCERT Solution
|
|
|
Study material: Topic- Empirical formula and molecular formula
|
|
|
Practice Material: CBSE Board Question banks
|
|
|
CBSE Solved Test Paper
|
|
|
CBSE Solved Paper
|
|
|
Solved Exam accelerator important Questions
|
|
|
Discovery of a fundamental particle protons
|
|
|
Discovery of the neutron (Structure of Atom)
|
|
|
How was the Discovery of Electron? (Structure of Atom)
|
|
|
Class IX Chemistry E-Notes Chapter Atomic Structure
|
|
|
IX Quick revision E Notes for chapter Structure of Atom
|
|
|
Discovery of the Neutron - Ruther ford Model of Atom
|









No comments:
Post a Comment