Michale Faraday Studied the effect of electricity on solutions, coined term "electrolysis" as a splitting of molecules with electricity, developed laws of electrolysis.
In 1859, J. Plucker Built one of the first gas discharge tubes ("cathode ray tube").
It was George Johnstone Stoney, an Irish Physicist who first proposed the word ‘electron‘ for atom of electricity’ in 1891.His contribution to research in this area laid the foundations for the eventual discovery of particles by J.J. Thomson in 1897.
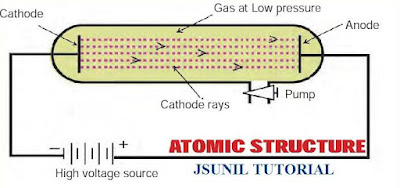
In 1878, Sir William Crooke, while conducting an experiment using a discharge tube, found certain visible rays travelling between two metal electrodes. These rays are known as Crooke’s Rays or cathode rays. The discharge tube used in the experiment is now referred to as Crookes tube or more popularly as Cathode Ray Tube (CRT).
Cathode Ray Tube : It is a long glass tube filled with gas and sealed at both the ends. It consist of two metal plates (which act as electrodes) connected with high voltage. The electrode which is connected to the negative terminal of the battery is called the cathode (negative electrode). The electrode connected to the positive terminal is called the anode (positive electrode). There is a side tube which is connected to a pump. The pump is used to lower the pressure inside the discharge tube.
Discovery of electron
Later, J.J. Thomson also found that when a high voltage of 10,000 V was applied between the electrodes present in a partially evacuated cathode ray tube at a pressure of 0.01mm of mercury, a bright spot of light was formed on the screen coated with a fluorescent material placed at the other end of the tube.
The Fluorescent material coated on the screen started to glow because it was struck by the ray which originated from the cathode. Since these rays were emitted by the cathode, he named these rays as cathode rays. Later, he named it as electrons.
Properties of cathode rays
1.The cathode rays travel in straight lines.
Cathode rays fall on a small object which is placed in between the cathode and anode.A shadow which is of the same shape as the object is observed on the wall opposite to the cathode.
2.Cathode rays are made up of small particles that have mass and kinetic energy.
Cathode rays fall on a light paddle wheel which is placed between cathode and anode. The wheel starts rotating.
3.The cathode rays are negatively charged particles
Cathode rays are passed through an electric field. The cathode rays are deflected towards the positive plate of electric field.
4. Cathode rays are passed through a magnetic field . The deflection of the rays is perpendicular to the applied magnetic field.
The direction of deflection indicates that the cathode rays constitute negatively charged particles. These negatively charged particles are called electrons
Characteristics of cathode rays :
1) Cathode rays are not visible but their behavior can be observed with the help of a fluorescent or a phosphorescent.
2) These rays travel from cathode to anode.
3) These rays travel in straight lines in the absence of electric and magnetic fields.
4) However these rays are deflected towards the positive end in electric field. Hence it was concluded that the rays constitute negatively charged particles and are known as electrons.
These rays are also deflected in magnetic field.
5) The behavior and the properties of rays are independent of the nature of the cathode material and nature of the gas present in the cathode ray tube.
These facts conclude that the electrons are the negatively charged fundamental particles present in all the substances.
Charge to mass ratio of Electron (e/me):
The charge to mass ratio of electron was calculated by J. J. Thomson. Its value is equal to 1.75882x10^11C.kg-1.
Charge on the Electron (e) : The charge on the electron was calculated by Millikan in oil drop experiment as 1.60 x 10-19 coulombs. Now the mass of an electron can be derived as follows:
Related Post -Structure of Atom: IX
Related Post -Structure of Atom: IX
Visit to Download Related Post -Structure of Atom
|
|
Structure of Atom: IX NCERT Solution
|
|
Study material: Topic- Empirical formula and molecular formula
|
|
Practice Material: CBSE Board Question banks
|
|
CBSE Solved Test Paper
|
|
CBSE Solved Paper
|
|
Solved Exam accelerator important Questions
|
|
Discovery of a fundamental particle protons
|
|
Discovery of the neutron (Structure of Atom)
|
|
How was the Discovery of Electron? (Structure of Atom)
|
|
Class IX Chemistry E-Notes Chapter Atomic Structure
|
|
IX Quick revision E Notes for chapter Structure of Atom
|
|
Discovery of the Neutron - Ruther ford Model of Atom
|












No comments:
Post a Comment